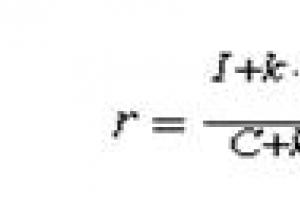Homemade rocket on gasoline. How to make rocket fuel at home. Of all the arts
A few decades ago, when humanity was raving about space exploration, rocket science was rampant. Both schoolchildren and adult men enthusiastically designed in garages and kitchens from improvised materials. Now the hype has subsided a bit, but what could be more exciting than launching your own made aircraft into the air? How to make a rocket take off? The most accessible and practical is to use caramel fuel, a mixture of saltpeter and carbohydrate.
What will be required
The set of components is not so great.
1. Sugar or sorbitol - raw material for caramelization.
2. Saltpeter (you can use different ones, more on that below).
3. Metal container - most often they take ordinary cans, although it is preferable to take dishes with thick walls - for more uniform heating. Even better - enameled or stainless steel, so that there is no reaction of the solution with the material of the dishes.
4. Electric stove - cook fuel on gas stove it is forbidden!
5. Newspaper or other paper with good absorbent properties (if your goal is to make not just caramel fuel, but caramel paper). It is also used in rocket engines, impregnated with ready-made "caramel" and dried (without heating).
6. Protective equipment: goggles and gloves.
7. Ventilation.
Three manufacturing methods
You can make caramel fuel in different ways. The easiest thing is to just mix the ingredients. Another "caramel" is boiled - simply or with evaporation. With normal mixing, the fuel is poured into a glass jar and shaken several times, then tightly closed to prevent water absorption. When used directly in rocket engines, this type of fuel must be well compacted, otherwise an explosion is possible.

Boil, or rather, melt caramel fuel at a temperature of 120-145 degrees until the sugar is completely converted and a mass is formed that is similar in consistency to liquid semolina. It is not necessary to pre-grind the components. It is very important to constantly stir it so that air bubbles do not form. Evaporation cooking involves adding water and then evaporating it. The disadvantages of this method: moisture remains in the fuel, and this reduces the rate of its combustion.
Recipe #1
Caramel fuel from is the best option. The ingredients are taken in the following proportions: sugar or sorbitol - 35%; saltpeter - 65%. Saltpeter is dried in a flat wide frying pan about 100-150 degrees for about two hours. Then grind for about 20 seconds - you can use a mortar or coffee grinder.

Lay in equal portions, 50 grams each. In order not to bother with grinding sugar, it is better to buy ready-made powdered sugar. For "boiled" caramel fuel, nothing needs to be ground or dried. To increase efficiency, 1% iron oxide (Fe 2 O 3) can be added to the mixture.
Recipe number 2
Caramel fuel from sodium nitrate. Features of this mixture - it is more hygroscopic. It will take 70% saltpeter, 30% sugar and two volumes of water (200%).
Recipe number 3
It is not recommended to use it. fuel for (ammonium nitrate). Why is it better to pay attention to other recipes? Because this is an unstable connection, and when heated, anything can go wrong. As a result, the idea is likely to end in a fire!

In addition, in the manufacture of "caramel" from ammonium nitrate highly toxic fumes are released. Therefore, all recipes using ammonium nitrate contain additional components to convert it to sodium or potassium. The easiest option is with sodium. We take 40% saltpeter, 45% baking soda and 200% water. We note the liquid level and evaporate until the smell of ammonia disappears. Then we add water to the original level (it has also partially evaporated), add 15% sugar and wait for it to dissolve.
Catalysts
To increase the effectiveness of "caramel", various catalysts are added to it. The most popular is iron oxide. Less known caramel fuel with aluminum. Attention! A mixture of aluminum and nitrates may ignite in the presence of water. Especially dangerous is the presence of any alkaline impurities that may be present in saltpeter that is not pure enough or made by yourself. Therefore, in a fuel based on nitrates with aluminum as a catalyst, it is necessary to add 0.5-1% of some weak acid, and it is not a fact that this amount is enough - it all depends on the quality of the saltpeter. Boric - the best option. Oxalic and acetic are not suitable - aluminum reacts with them. If during the cooking process the mixture becomes very hot, foams and emits a strong smell of ammonia, you must immediately remove it from the stove and immerse it in water.

In general, it is better for experienced rocket scientists who have mastered the simplest types of fuel to experiment with catalysts. Yes, and it doesn’t hurt to learn chemistry: it’s easy to use ready-made tips, but knowledge and understanding of what you are doing and what reactions occur in the mixture is much more valuable.
Aluminum is added to the potassium caramel. Permissible variations are from 2.5 to 20%. A different amount gives a different change in the burning rate of the fuel. It is recommended to use spherical aluminum ASD-4.
How to stay whole and healthy
The most dangerous way to prepare caramel fuel is by melting sugar and saltpeter, but this option is also the most effective. The container in which the "caramel" is boiled must be perfectly clean - foreign substances can cause a fire.
There should not be sources of open flame nearby - we don't need explosions in the kitchen. It is very important to monitor the temperature of the mixture - it should not rise above 180 degrees under any circumstances!

When stirring, it is better to use a wooden stick to avoid adverse reactions. It should be mixed very carefully, but evenly: air bubbles in the finished fuel, when used, lead to an explosion of the rocket. When pouring this fuel into molds, you also need to make sure that there are no bubbles. It is necessary to work with a hood or on fresh air, especially for the recipe with ammonium nitrate.
Do not grind sugar and saltpeter together in a coffee grinder! You need to grind separately, mix, shaking, in a glass bowl.
Beginners should not mess with ammonium nitrate: first try the simplest and safest (based on potassium nitrate) caramel fuel. The manufacture of any homemade fuel must take place under the most careful control of the quality of ingredients, temperature, moisture content and in compliance with all safety measures!
Where to get the ingredients
Saltpeter is sold in agricultural supply stores and departments for summer residents as a fertilizer. Sorbitol is a sugar substitute for diabetics. Sold, respectively, in a pharmacy. Fe 2 O 3 - iron oxide - used to be sold under the name You can try to make it yourself by studying the relevant literature. Mineral hematite - this is also aluminum sold by chemical reagent manufacturers.
The disadvantages of this fuel compared to conventional sorbitol are: difficulty in manufacturing, low ductility, impossibility of pouring the composition into the engine housing, fast solidification rate, with insufficient heating of sorbitol, the fuel quickly solidifies. Experience has shown that this fuel is well prepared and used in the cold season, since the humidity in the air is much lower than in summer time. Perhaps the most main problem of this fuel is the fast solidification rate and the impossibility of pouring fuel directly into the engine housing. This fuel also has a very unpleasant thing - if the mass is not compacted enough, voids form inside the fuel charge, which greatly affects the uniformity of combustion of the entire charge. Simply put, the structure becomes porous, which contributes to the occurrence of abnormal combustion - an unstable intermittent combustion caused by a decrease in heat supply to unreacted fuel, lasting from a few fractions to 2 seconds. This problem is especially typical only for small engines, with a fuel charge of 30 - 35 grams - pressing "Powerful Caramel" into such engines is a very painstaking and complicated work, but such a thing has practically no effect on large engines, because with respect to the entire volume of fuel air voids are small. Although this fuel solidifies quickly, this problem can be easily eliminated by placing a container of fuel in a heated sand bath. This is a very convenient way, well, don't overdo it with the temperature, otherwise the sulfur in the fuel will melt and the mixture will become inhomogeneous.
MANUFACTURING
At first, there were serious problems in its manufacture. It was difficult to find a balance between the melting point of sorbitol and the melting point of sulfur, and when the melts of both components were mixed, the fuel was extremely inhomogeneous. A variant was considered using glycerin so that the mass retains plasticity long time. But the use of glycerin led to a decrease in the strength of the fuel pellet and increased hygroscopicity.
Sorbitol, when heated strongly and then cooled, does not solidify immediately and retains its plasticity for quite a long time, which is enough to refuel 2-3 small engines. Sorbitol must be heated to sufficient high temperature(about tbp). When I heat it up to this temperature, it smokes a little, becomes transparent (slightly yellowish), and small bubbles form at the bottom, which indicates the beginning of the boil.
Before you start melting sorbitol, you should prepare all the components in advance.
1. First, weigh out the required portion of sorbitol and put it away from the place of work
Before you start melting sorbitol, you should prepare all the components in advance
2. Next, you will need to grind the potassium nitrate. Before grinding, it should be thoroughly dried, it is possible on a battery, but I dried it in an oven at t ≈ 2000C, it is impossible to exceed this temperature, because melting begins and then decomposition. Dried potassium nitrate grinds more easily and sticks less to the walls of the electric coffee grinder than wet. I grinded it in an electric coffee grinder for about 40 seconds. If it stuck to the walls, then it can be scraped off with cotton swabs or hands, but not bare, but using disposable gloves.
Next, you will need to grind potassium nitrate
I grinded in an electric coffee grinder for about 40 seconds
3. After grinding, weigh out the required portion of saltpeter and place in a clean jar, I used a plastic one, because. It sticks to my glass.
After grinding, weigh out the required portion of saltpeter and place in a clean jar
4. Then you need to weigh the sulfur.
Then you need to weigh the sulfur
The sulfur that I use in fuel contains coal in the following ratio: 100% (S) + 5% (C) (by mass).
When using coal, the mass forms fewer lumps, becomes more crumbly and practically does not stick to the walls of the electric coffee grinder during grinding. However, it is necessary to grind intermittently so that the sulfur does not melt from excessive friction. After grinding, it remains highly electrified and will form lumps. As I noted, it takes quite a long time for the sulfur to become crumbly after grinding, so it should be milled in advance.
5. Only after you have measured everything can you melt the sorbitol. For these purposes, I used my favorite miniature oven, but when I didn’t have one, I made do with a stove. Sorbitol is placed in metal container, but better in a stainless steel container (I personally use a stainless steel mug, which I purchased at the Vse for Fishing and Hunting store) and heats up to a temperature close to its boiling point.
Only after you have measured everything can you melt the sorbitol
6. Then finely ground and dried potassium nitrate (potassium nitrate) is added to it. Before you fall asleep, shake the vial of saltpeter well so that it becomes more crumbly.
Then finely ground and dried potassium nitrate (potassium nitrate) is added to it.
7. The mixture is stirred until completely homogeneous. With this ratio of saltpeter and sorbitol, the mixture begins to solidify quickly, so you will have to reheat the contents of the glass until the mixture is ready to stir.
The mixture is stirred until completely homogeneous.
8. After the mixture has cooled to a temperature that is below the melting point of sulfur, sulfur itself is added to it. The temperature can be checked by dropping a small amount of sulfur into the above mixture of saltpeter and sorbitol, if the temperature is too high, the sulfur will melt and form small, shiny droplets on the surface. Mix all the ingredients very quickly so that the mixture does not have time to harden.
After the mixture has cooled to a temperature that is below the melting point of sulfur, sulfur itself is added to it.
10. After that, pull out the plastic mass (it is advisable to use disposable polyethylene gloves) with a knife or other metal object. The mixture should also be scraped off the walls of the mug and kneaded again with your hands for greater uniformity (use plastic gloves!).
I want to note that the fuel begins to solidify quickly, so I place it again in a mug and put it in a heated oven, but only turned off, because. it retained heat in itself and perfectly helps to maintain the temperature of the fuel melt and it does not remain plastic for a long time. You can also put some heat-intensive materials into the oven: clean dry sand, metal nuts, nails, lead is perfect. As necessary, pieces of fuel are plucked from the main mass and carefully pressed into the engine housing.
After that, pull out the plastic mass (it is advisable to use disposable polyethylene gloves) with a knife or other metal object
The fuel should be pressed in in small portions, because if the fuel is not pressed under sufficient pressure, then many air bubbles will remain inside the fuel block. As experience has shown, for pressing it is better to use a graphite stick impregnated with paraffin, and with a polished tip. For these purposes, fluoroplastic is also suitable, but the fuel still sticks to it and it is advisable to have a rag on hand with which you will remove plaque. All work should preferably be carried out in a dry room. As I have already noted, this fuel is more suitable for the manufacture of large fuel charges (from 70g) for large engines.
Author's note: I don't know if this fuel will become popular with rocket scientists and chemists, but after working with it for a long time, I have come to the conclusion that this is the only powerful fuel that can be obtained without much difficulty compared to perchlorate. And the lower sorbitol content makes it a little more profitable to use, unless of course your sulfur is cheaper than sorbitol. From the first time, you will not be able to cook it the way you need it, but in the course of long work with it, you will really see the difference. It may seem to you that this way the manufacture of this fuel is unsafe, but in all my practice there has not been a single emergency, because I strictly observe the purity of reagents and do not allow substances that ignite below 2000C to enter. With strict observance of the cleanliness of the workplace, this method is relatively safe.
| | | | r-s | t-y | f-ts | sh-i
Composition No. 1: 60% (9KNO 3) + 30% (9SORBIT) + 10% (9S) 9 - higher plasticity
Composition No. 2: 63% (KNO 3) + 27% (SORBIT) + 10% (S) - maximum specific thrust
This propellant is a new and much improved version of sorbitol propellant. Its faster burn rate and high specific impulse make it suitable for use in both medium and large rocket engines. It was developed by me recently, i.e. improved, because It was not my idea to use sorbitol as a binder. However, compositions similar to it have been published on some web pages of the Internet. But they never became popular with rocket scientists. And I think you know why.
The composition of the new sorbitol fuel includes sulfur, which is involved in the combustion reaction:
6C 6 H 14 O 6 + 26KNO 3 + 13S = 13K 2 S + 36CO 2 + 13N 2 + 42H 2 O (theoretically)
In fact, the reaction proceeds according to a more complex mechanism, according to the redox properties of the elements, it can be argued that at the very beginning, the reaction will proceed precisely according to a simple mechanism, and only then the reaction products will interact with each other, giving already other compounds. The correct ratio of components ensures the high efficiency of this fuel. This fuel has relatively high energy characteristics. The fact is that sulfur is involved here as a reducing agent and displaces the remaining oxygen atom from the molecule K2O, resulting in an increase in the energy yield of the reaction. Besides K 2 S does not pick up CO2 how does it do K2O. The released energy is enough to shift the equilibrium towards the formation of such low molecular weight products as CO And H2. This contributes to a significant increase in the specific thrust of the fuel. Thus, the efficiency of the engine increases on average by 15 - 20% (according to rough estimates), and maybe more. So we can say that this rocket fuel is a worthy replacement for gunpowder and ordinary caramel.
The disadvantages of this fuel compared to conventional sorbitol are: difficulty in manufacturing, low ductility, impossibility of pouring the composition into the engine housing, fast solidification rate, with insufficient heating of sorbitol, the fuel quickly solidifies. Experience has shown that this fuel is well prepared and used in the cold season, since the humidity in the air is much lower than in summer. Perhaps the most important problem with this fuel is the rapid solidification rate and the impossibility of pouring fuel directly into the engine housing. This fuel also has a very unpleasant thing - if the mass is not compacted enough, voids form inside the fuel charge, which greatly affects the uniformity of combustion of the entire charge. Simply put, the structure becomes porous, which contributes to the formation abnormal burning- unstable intermittent combustion caused by a decrease in heat supply to unreacted fuel, lasting from a few fractions to 2 seconds. This problem is especially characteristic only for small engines, with a fuel charge 30 - 35 grams- pressing "Powerful caramel" into such engines - the work is very painstaking and complicated, but such a thing has practically no effect on large engines, because air voids are insignificant relative to the entire volume of fuel. Although this fuel solidifies quickly, this problem can be easily eliminated by placing a container of fuel in a heated sand bath. This is a very convenient way, well, don't overdo it with the temperature, otherwise the sulfur in the fuel will melt and the mixture will become inhomogeneous.
MANUFACTURING
At first, there were serious problems in its manufacture. It was difficult to find a balance between the melting point of sorbitol and the melting point of sulfur, and when the melts of both components were mixed, the fuel was extremely inhomogeneous. A variant was considered using glycerin, so that the mass retains plasticity for a long time. But the use of glycerin led to a decrease in the strength of the fuel pellet and increased hygroscopicity.
Sorbitol with strong heating and subsequent cooling does not harden immediately and retains plasticity for a sufficiently long time, which is enough for refueling 2 - 3 small engines. Sorbitol must be heated to a sufficiently high temperature (about t kip). When I heat it up to this temperature, it smokes a little, becomes transparent (slightly yellowish), and small bubbles form at the bottom, which indicates the beginning of the boil.
Before you start melting sorbitol, you should prepare all the components in advance.
1. First, weigh out the required portion of sorbitol and put it away from the place of work

2. Next, you will need to grind the potassium nitrate. Before grinding, it should be thoroughly dried, it is possible on the battery, but I dried it in the oven at t ≈ 200 0 C, more than this temperature is impossible, because melting begins and then decomposition. Dried potassium nitrate grinds more easily and sticks less to the walls of the electric coffee grinder than wet. I grinded in an electric coffee grinder for about seconds 40 . If it sticks to the walls, then it can be scraped off with cotton swabs or hands, but not bare, but using disposable gloves.


3. After grinding, weigh out the required portion of saltpeter and place in a clean jar, I used a plastic one, because. It sticks to my glass.


The sulfur that I use in fuel contains coal in the following ratio: 100% (S) + 5% (C) (by mass).
When using coal, the mass forms fewer lumps, becomes more crumbly and practically does not stick to the walls of the electric coffee grinder during grinding. However, it is necessary to grind intermittently so that the sulfur does not melt from excessive friction. After grinding, it remains highly electrified and will form lumps. As I noted, it takes quite a long time for the sulfur to become crumbly after grinding, so it should be milled in advance. ()
5. Only after you have measured everything can you melt the sorbitol. For these purposes, I used my favorite miniature oven, but when I didn’t have one, I made do with a stove. Sorbitol is placed in a metal container, and preferably in a stainless steel container (I personally use a stainless steel mug that I purchased in a store "All for fishing and hunting") and is heated to a temperature close to its boiling point.


6. Then finely ground and dried potassium nitrate (potassium nitrate) is added to it. Before you fall asleep, shake the vial of saltpeter well so that it becomes more crumbly.

7. The mixture is stirred until completely homogeneous. With this ratio of saltpeter and sorbitol, the mixture begins to solidify quickly, so you will have to reheat the contents of the glass until the mixture is ready to stir.

8. After the mixture has cooled to a temperature that is below the melting point of sulfur, sulfur itself is added to it. The temperature can be checked by dropping a small amount of sulfur into the above mixture of saltpeter and sorbitol, if the temperature is too high, the sulfur will melt and form small, shiny droplets on the surface. Mix all the ingredients very quickly so that the mixture does not have time to harden.

10. After that, pull out the plastic mass (it is advisable to use disposable polyethylene gloves) with a knife or other metal object. The mixture should also be scraped off the walls of the mug and kneaded again with your hands for greater uniformity (use plastic gloves!).
I want to note that the fuel begins to solidify quickly, so I place it again in a mug and put it in a heated oven, but only turned off, because. it retained heat in itself and perfectly helps to maintain the temperature of the fuel melt and it does not remain plastic for a long time. You can also put some heat-intensive materials into the oven: clean dry sand, metal nuts, nails, lead is perfect. As necessary, pieces of fuel are plucked from the main mass and carefully pressed into the engine housing.

The fuel should be pressed in in small portions, because if the fuel is not pressed under sufficient pressure, then many air bubbles will remain inside the fuel block. As experience has shown, for pressing it is better to use a graphite stick impregnated with paraffin, and with a polished tip. For these purposes, fluoroplastic is also suitable, but the fuel still sticks to it and it is advisable to have a rag on hand with which you will remove plaque. All work should preferably be carried out in a dry room. As I already noted, this fuel is more suitable for the manufacture of large fuel charges (from 70g) for large engines.
From the author: I don't know if this fuel will become popular with rocket scientists and chemists, but after working with it for a long time, I have come to the conclusion that this is the only powerful fuel that can be obtained without much difficulty compared to perchlorate. And the lower sorbitol content makes it a little more profitable to use, unless of course your sulfur is cheaper than sorbitol. From the first time, you will not be able to cook it the way you need it, but in the course of long work with it, you will really see the difference. It may seem to you that this method of making this fuel is unsafe, but in all my practice there has not been a single state of emergency, because I strictly observe the purity of reagents and do not allow substances that ignite below 2000C. With strict observance of the cleanliness of the workplace, this method is relatively safe.
Attention! If you have any comments, questions or suggestions on this topic, please let me know.
Sometimes you want something weird. So, recently I was drawn to rocket modeling. Since I build rockets at the noob level, for me a rocket consists of two parts - an engine and a body. Yes, I know that everything is much more complicated, but even with this approach, rockets fly. Naturally, you are interested in how the engine is made.
I want to warn you that if you are going to repeat what is written in this article, you will do it at your own peril and risk. I do not guarantee the accuracy or safety of the proposed technique.
For the motor housing, I use thick-walled PVC pipes 3/4 inch diameter. Pipes of this diameter are relatively cheap and widely available. Pipes are best cut with special scissors. I suffered a lot, trying to cut such pipes with an electric jigsaw - it always turned out very crooked.

I mark the pipe like this:

All dimensions are in inches. who does not know, the size in inches must be multiplied by 2.54 and you get the size in centimeters. These dimensions I found in a wonderful book
There are a bunch of other designs out there as well. The upper piece of the engine (which is empty) I do not. There should be an expelling charge for the parachute, I'm still far from that.
The cut piece of pipe is inserted into a special fixture. I will show all the adaptations at once, so that there are no questions:

A long stick plays the role of a “pestle.” Clay and fuel are compacted with it. The second detail is the conductor. It serves to drill a nozzle exactly in the center of the engine. Here are their drawings:

The drill is used long - 13cm long. It is just enough to drill a channel through all the fuel.
Now you need to mix the fuel. I use the standard "caramel" - sugar and saltpeter in the ratio of 65 saltpeter / 35 sugar. I don’t want to melt caramel - this is a risky occupation, and it’s not worth that hemorrhoids. I'm not trying to get the best out of the fuel. It's amateur rocket science. I just mix powdered sugar and saltpeter in powders:


We hammer the powder according to the markup. You have to hit pretty hard.

Clogging fuel and plugs is no different. It seems that it is dangerous to knock on fuel, but caramel is difficult to ignite even from a match. Naturally, it is worth observing the basic precautions - do not bend over the engine, work in a protective mask, etc.
I leave the last 5mm plugs for hot melt adhesive. I tried several times to make a rocket without a hot melt plug, the top plug was torn out by pressure. Hot glue has excellent adhesion to plastic and does not have time to melt when the engine burns.
We drill the nozzle through the conductor:

Fuel is very poorly drilled - sugar melts and sticks to the drill, so it often has to be pulled out and the stuck fuel removed. Checking the nozzle:

We fill the last 5mm of the tube and its end with hot glue
 Everything, the engine is ready. This is what the engine looks like on static tests. Unfortunately, the video is not indicative - in this engine, the channel was half-drilled, and the camera did not record the sound correctly. In real life, the “roar” of the engine is very loud and serious, and not as toy as on the record.
Everything, the engine is ready. This is what the engine looks like on static tests. Unfortunately, the video is not indicative - in this engine, the channel was half-drilled, and the camera did not record the sound correctly. In real life, the “roar” of the engine is very loud and serious, and not as toy as on the record.
Few of my peers were not fond of building model rockets. Maybe it was the worldwide passion of mankind for manned flights, or maybe the apparent simplicity of building a model. A cardboard tube with three stabilizers and a foam or balsa head fairing, you see, is much simpler than even an elementary model of an airplane or car. True, the enthusiasm of most young Korolevs, as a rule, evaporated at the stage of searching for a rocket engine. The rest had no choice but to master the basics of pyrotechnics.
Alexander Grek
Between the Chief Designer of our rockets, Sergei Korolev, and the Chief Designer of our rocket engines, Valentin Glushko, there was a tacit struggle for the title of the Most Important: who is really more important, the designer of rockets or engines for them? Glushko is credited with a catchphrase allegedly thrown by him in the midst of such a dispute: “Yes, I will tie a fence to my engine - it will go into orbit!” However, these words are by no means empty boasting. The rejection of the "Glushkov" engines led to the collapse of the royal lunar rocket H-1 and deprived the USSR of any chance of winning the lunar race. Glushko, having become the general designer, created the Energiya super-powerful launch vehicle, which no one has been able to surpass so far.

Cartridge engines
The same pattern worked in amateur rocket science - a rocket with a more powerful engine flew higher. Despite the fact that the first model rocket engines appeared in the USSR even before the war, in 1938, Evgeny Buksh, the author of the book “Fundamentals of Rocket Modeling”, published in 1972, took the cardboard sleeve of a hunting cartridge as the basis for such an engine. The power was determined by the caliber of the original sleeve, and the engines were produced by two pyrotechnic workshops of DOSAAF until 1974, when a decision was made to organize rocket modeling sports in the country. To participate in international competitions, engines were required that were suitable in their parameters for the requirements of the international federation.
Their development was entrusted to the Perm Research Institute polymer materials. Soon an experimental batch was released, on the basis of which the Soviet rocket modeling sport began to develop. Since 1982, serial production of engines has been intermittently launched at the Impulse state-owned state-owned plant in Ukrainian Shostka - 200-250 thousand copies were produced per year. Despite the severe shortage of such engines, this was the heyday of Soviet amateur model rocketry, which ended in 1990 simultaneously with the closure of production in Shostka.
Engine tuning
The quality of serial engines, as you might guess, was not suitable for serious competitions. Therefore, in 1984, a small-scale pilot production appeared next to the plant, providing the national team with its products. Particularly distinguished were the engines, privately made by the master Yuri Gapon.

And what, in fact, is the complexity of production? At its core, a model rocket engine is the simplest device: a cardboard tube with DRP-3P black powder pressed inside (smoky gunpowder 3rd composition for pressed products) with a ceramic plug with a hole nozzle on one side and a wad with an expelling charge on the other . The first problem that mass production could not cope with was the accuracy of the dosage, on which the final total impulse of the engine depended. The second is the quality of the hulls, which often cracked when pressed under a pressure of three tons. Well, the third - in fact, the quality of pressing. However, quality problems arose not only in our country. The serial model rocket engines of another great space power, the United States, do not shine with them either. And the best model engines are made by microscopic enterprises in the Czech Republic and Slovakia, from where they are smuggled for especially important events.
Nevertheless, under socialism, engines, albeit unimportant and in short supply, were. Now they don't exist at all. Separate children's rocket modeling studios fly on old, still Soviet stocks, turning a blind eye to the fact that the expiration date has long passed. Athletes use the services of a couple of lone masters, and if you're lucky, then smuggled Czech engines. The only way left for amateurs is to become Glushko first before becoming the Queen. That is, to make the engines themselves. What, in fact, did I and my friends in childhood. Thank God, everyone's fingers and eyes remained in place.
Of all the arts
Of all the arts, cinema is the most important for us, Ilyich liked to say. For rocket modellers-amateurs of the middle of the last century - too. For the film and photographic film of that time was made of celluloid. Tightly rolled up into a small roll and stuffed into a paper tube with stabilizers, it allowed a simple rocket to fly up to the height of a five-story building. Such engines had two main drawbacks: the first was low power and, as a result, flight altitude; the second is the non-renewability of celluloid film stocks. For example, my father's photo archive was only enough for a couple of dozen launches. Now, by the way, it's a pity.
 The maximum height at a fixed total engine impulse was achieved with a short-term four-fold power jump at the start and a further transition to an even average thrust. The thrust jump was achieved by forming a hole in the fuel charge.
The maximum height at a fixed total engine impulse was achieved with a short-term four-fold power jump at the start and a further transition to an even average thrust. The thrust jump was achieved by forming a hole in the fuel charge.
The second version of the engines was assembled, so to speak, from the waste products of the Soviet army. The fact is that when firing at artillery ranges (and one of them was just not far from us), the propellant charge does not completely burn out when fired. And if you searched carefully in the grass in front of the positions, you could find quite a lot of tubular gunpowder. The simplest rocket was obtained by simply wrapping such a tube in ordinary foil from a chocolate bar and igniting it at one end. Such a rocket flew, however, low and unpredictable, but fun. Powerful engine was obtained by collecting long tubes in a bag and pushing them into a cardboard case. A primitive nozzle was also made from baked clay. Such an engine worked very effectively, raised the rocket quite high, but often exploded. In addition, you don’t really look like an artillery range.

The third option was an attempt at almost industrial production of a rocket engine using homemade black powder. They made it from potassium nitrate, sulfur and activated carbon(he constantly jammed the parent coffee grinder, on which I ground him to dust). To be honest, my powder engines worked intermittently, lifting the rockets only a couple of tens of meters. I found out the reason only a couple of days ago - it was necessary to press in the engines not with a hammer in the apartment, but with a school press in the laboratory. But who, one wonders, would have let me press in rocket engines in the seventh grade?!
 Two of the rarest engines that PM managed to get: MRD 2, 5-3-6 and MRD 20-10-4. From the Soviet stocks of the rocket-modeling section in the Children's House of Creativity on Sparrow Hills.
Two of the rarest engines that PM managed to get: MRD 2, 5-3-6 and MRD 20-10-4. From the Soviet stocks of the rocket-modeling section in the Children's House of Creativity on Sparrow Hills.
Working with poisons
The pinnacle of my engine-building activity was a rather poisonous engine that ran on a mixture of zinc dust and sulfur. I traded both ingredients from a classmate, the son of the director of the city pharmacy, for a pair of rubber Indians, the most convertible currency of my childhood. I got the recipe from a terribly rare translated Polish rocket model book. And I stuffed the engines in my father's gas mask, which we kept in the pantry - in the book, special emphasis was placed on the toxicity of zinc dust. The first trial run was carried out in the absence of parents in the kitchen. A column of flame from the engine, clamped in its vice, roared up to the ceiling, smoking a spot on it with a diameter of a meter and filling the apartment with such smelly smoke that even a box of smoked cigars cannot be compared with. It was these engines that provided me with record launches - probably fifty meters. Imagine my disappointment when, twenty years later, I found out that the children's rockets of our scientific editor Dmitry Mamontov flew many times higher!
 1, 2, 4) In the presence of a factory rocket engine, a primary school student can also cope with the construction of a simple rocket. 3) A product of amateur creativity - an engine from a cartridge case.
1, 2, 4) In the presence of a factory rocket engine, a primary school student can also cope with the construction of a simple rocket. 3) A product of amateur creativity - an engine from a cartridge case.
On fertilizers
Dmitry's engine was simpler and more technologically advanced. The main component of his rocket fuel is sodium nitrate, which was sold in hardware stores as fertilizer in 3 and 5 kg bags. Saltpeter served as an oxidizing agent. And an ordinary newspaper acted as a fuel, which was soaked in a supersaturated (hot) solution of saltpeter, and then dried. True, during the drying process, saltpeter began to crystallize on the surface of the paper, which led to a slowdown in combustion (and even extinction). But here know-how came into play - Dmitry ironed the newspaper with a hot iron, literally fusing saltpeter into paper. It cost him a damaged iron, but such paper burned very quickly and stably, releasing a large amount of hot gases. Stuffed with nitrate paper rolled into a tight roll cardboard tubes with improvised nozzles from bottle caps, they flew up to a hundred or two meters.
Caramel
The paranoid ban of the Russian authorities on the sale to the population of various chemicals from which explosives can be made (and it can be made from almost anything, even from sawdust), is offset by the availability of online recipes for almost all types of rocket fuel, including, for example, the composition of fuel for the Shuttle boosters (69.9% ammonium perchlorate, 12.04% polyurethane, 16% aluminum powder, 0.07% iron oxide and 1.96% hardener).
 Cardboard or foam shells of rockets, propellants based on gunpowder do not seem to be very serious achievements. But who knows - maybe these are the first steps of the future designer of interplanetary ships?
Cardboard or foam shells of rockets, propellants based on gunpowder do not seem to be very serious achievements. But who knows - maybe these are the first steps of the future designer of interplanetary ships?
The undisputed hit of amateur rocket engine building is now the so-called caramel engines. The fuel recipe is obscenely simple: 65% KNO3 potassium nitrate and 35% sugar. Saltpeter is dried in a frying pan, after which it is ground in an ordinary coffee grinder, slowly added to melted sugar and solidifies. The result of creativity is fuel checkers, from which you can recruit any engines. Spent cartridge cases from hunting cartridges are perfect as engine cases and forms - hello to the thirties! Sleeves in unlimited quantities are on any shooting stand. Although recognized masters recommend using not sugar, but sorbitol caramel in the same proportions: sugar develops more pressure and, as a result, inflates and burns through the sleeves.

Back to the Future
The situation, one might say, returned to the 1930s. Unlike other modeling sports, where the lack of domestic engines and other components can be compensated by imports, this does not work in rocket modeling sports. In our country, model rocket engines are equated with explosives, with all the ensuing conditions for storage, transportation and transportation across the border. A person capable of importing such products has not yet been born on Russian soil.
There is only one way out - production at home, since the technology here is not at all space. But factories that have licenses for the production of such products do not undertake them - they would be interested in this business only with millions of copies. So novice rocket modellers from the largest space power are forced to fly on caramel rockets. Whereas in the United States, reusable model rocket engines have now begun to appear, running on hybrid fuel: nitrous oxide plus solid fuel. What country do you think will fly to Mars in thirty years?