What do aldehydes interact with? Aldehydes and ketones. Individual representatives of aldehydes and their meaning
The word aldehyde was coined as an abbreviation of the Latin alcohol dehydrogenatus - dehydrogenated alcohol, the most popular aldehyde is formaldehyde, resins are made from it, medicines are synthesized and as a preservative. The formula of an aldehyde is R-CHO, a compound in which the carbonyl group is bonded to a hydrogen and a radical.
The word ketone comes from the word acetone, the smallest compound in the ketone family. Ketones are used as solvents, drugs, and for the synthesis of polymers. The formula of a ketone is R-C(O)-R, a compound in which the carbonyl group is attached to two radicals.
Structure and properties of the carbonyl group
The carbonyl group is based on the bonding of a carbon atom and an oxygen atom through α- and π-bonds. The resonance structure of the group determines the high polarity of the compound and the electron cloud is shifted towards oxygen: C δ+ =O δ- . The introduction of electronegative elements into the bond reduces the polarity of the bond, increasing the positive charge of the molecule. Nucleophilic substituents increase the negative charge of oxygen.
The carbon atom in the carbonyl group is a strong electrophile (attaches electrons), so most reactions of aldehydes and ketones are carried out by nucleophilic reagents (Lewis bases). Logically, the oxygen atom is a strong nucleophile, and reactions with the oxygen atom are possible using electrophiles (Lewis acids).
Reaction of a carbonyl group with a Lewis base
(R)(R)C δ+ =O δ- + B: → (R)(R)C(B)-O
Reaction of a carbonyl group with a Lewis acid
(R)(R)C δ+ =O δ- + Y: → (R)(R)C-O-Y
In addition, oxygen's unshared electrons give it weak base properties, so those aldehydes and cetones that are insoluble in water will dissolve in concentrated sulfuric acid.
Physical properties of the carbonyl group
The high polarity of the C=O bond forms a high dipole moment, which is why carboxyl group carriers have a higher boiling point than hydrocarbons.
Unshared electrons in the oxygen atom form a hydrogen bond with water molecules, therefore, starting from five carbon atoms in the radicals, aldehydes and ketones are poorly soluble in water or do not dissolve at all.
Aldehydes and ketones with up to 12 carbon atoms are liquids. Aliphatic compounds with a carbonyl group have a density of about 0.8, therefore they float on the surface of water, cyclohexanone has a density of about unity, aromatic aldehydes and ketones have a density slightly higher than the density of water.
Reactions of aldehydes and ketones
Water connection
In the process of the reaction of water with aldehydes and ketones, diols (glycols, dihydric alcohols) are formed. The reaction proceeds using a catalyst - an acid or a base and is two-sided:
RR-CO + H-OH ↔ R R\ C /OH-OH
Addition of nucleophilic carbons
Important nucleophilic compounds that react with aldehydes and ketones are organometallic compounds (organic compounds in which there is a bond between a metal atom and a carbon atom/atoms). One of the representatives of organometallic compounds - Grignard reagents (general formula - R-Mg-X), in reactions with aldehydes and ketones form alcohols:
RH-C \u003d O + R-C - H 2 -Mg + -Cl - → RH-C- (O-MgCl) (CH 2 -R)
RH-C-(O-MgCl)(CH 2 -R) + H-OH → RH-C-CH 2 R + OH-Mg-Cl
Oxidation of aldehydes and ketones
When oxidized, aldehydes are intermediate between alcohols and carboxylic acids:
In the presence of hydrogen and oxygen:
R-CH 2 -OH ↔ R-C(=O)-H ↔ R-COOH
Aldehydes are readily oxidized, allowing the use of milder oxidants than simple oxygen. Aromatic aldehydes are more easily oxidized than aliphatic ones. The problem with the oxidation of aldehydes is the formation of by-products.
Ketones are difficult to oxidize; strong oxidizing agents and large amounts of heat are required to oxidize ketones. Breaks down as a result of oxidation C-C connection and an acid is formed (there is an exception):
In the presence of KMnO 4 , H and a large amount of heat :
CH 3 -C (= O) -CH 2 CH 3 → CH 3 -C (= O) -OH + CH 3 CH 2 -C (= O) -OH
An exception is the oxidation with selenium dioxide, SeO 2 , the methyl group following the carbonyl group is oxidized, converting to another carbonyl group. For example, methyl ethyl ketone is oxidized to diacetyl:
Oxidation of methyl ethyl ketone to diacetyl:
CH 3 CH 2 -C (= O) -CH 3 + SeO 2 → CH 3 -C (= O) -C (= O) -CH 3 + H 2 O + Se
The ease with which aldehydes are oxidized makes it easy to distinguish them from ketones; mild oxidizing agents are used for this, such as: Tollens' reagent (diammine silver hydroxide, Ag (NH 3) 2 OH), Fehling's reagent (an alkaline solution of copper ions Cu in Rochelle salt KNaC 4 H 6 O 6 4H 2 O) and Benedict's solution (copper ions with citrate and sodium carbonate). Aromatic aldehydes react with Tollens' reagent, but do not react with Benedict's and Fehling's reagents, which is used to determine the amount of aliphatic and aromatic aldehydes.
Polymerization of aldehydes
Paraldehyde
Acetaldehyde has a boiling point of 20°C, making it difficult to store and use. When acetaldehyde is treated with acid at low temperature, acetaldehyde combines into a cyclic triple molecule - paraldehyde, with a boiling point of 120°C. Paraldehyde depolymerizes on slight heating, releasing three molecules of acetaldehyde.
Formaldehyde
For ease of transportation and storage, formaldehyde is sold not in the form of gas, but in the form of formalin - an aqueous solution containing 37-40% paraformaldehyde, OH (CH 2 O) n H, with an average value of n = 30. Paraformaldehyde is a white amorphous solid obtained by slow evaporation of formalin at low pressure. Polymerization occurs due to the addition of formaldehyde molecules to each other:
CH 2 \u003d O + H 2 O ↔
+ n→ HO-(CH 2 O) n + 1 -H
Derlin polymer (polyoxymethylene) is a good linear plastic with high molecular weight, Derlin has excellent strength and elasticity characteristics.
(for the simplest aldehyde R=H)
Classification of aldehydes
According to the structure of the hydrocarbon radical:
Limit; For example:

Unlimited; For example:

Aromatic; For example:

Alicyclic; For example:

General formula of limit aldehydes

Homologous series, isomerism, nomenclature

Aldehydes are isomeric to another class of compounds - ketones

For example:

Aldehydes and ketones contain a carbonyl group ˃C=O, therefore they are called carbonyl compounds.
Electronic structure of aldehyde molecules
The carbon atom of the aldehyde group is in the state of sp 2 hybridization, so all σ-bonds in this group are located in the same plane. Clouds of p-electrons forming a π-bond are perpendicular to this plane and easily shift to the more electronegative oxygen atom. Therefore, the C=O double bond (unlike the C=C double bond in alkenes) is highly polarized.
Physical properties

Chemical properties
Aldehydes are reactive compounds that enter into numerous reactions. Most characteristic of aldehydes:
a) addition reactions at the carbonyl group; HX-type reagents are added as follows:

b) oxidation reactions C-H bonds aldehyde group, as a result of which carboxylic acids are formed:
I. Addition reactions
1. Hydrogenation (primary alcohols are formed

2. Addition of alcohols (hemiacetals and acetals are formed)

In an excess of alcohol in the presence of HCl, hemiacetals are converted to acetals:

II. Oxidation reactions
1. Silver mirror reaction

Simplified:

This reaction is a qualitative reaction to the aldehyde group (a mirror coating of metallic silver is formed on the walls of the reaction vessel).
2. Reaction with copper (II) hydroxide

This reaction is also a qualitative reaction to the aldehyde group y (a red precipitate of Cu 2 O precipitates).
Formaldehyde is oxidized by various O-containing oxidizing agents, first to formic acid and then to H 2 CO 3 (CO 2 + H 2 O):

III. Di-, tri- and polymerization reactions
1. Aldol condensation

2. Trimerization of acetaldehyde

3. Formaldehyde polymerization
During long-term storage of formalin (40% aqueous formaldehyde solution), polymerization occurs in it with the formation of a white precipitate paraform:

IV. Polycondensation reaction of formaldehyde with phenol

DEFINITION
Aldehydes- organic substances belonging to the class of carbonyl compounds containing in their composition the functional group -CH \u003d O, which is called carbonyl.
The general formula for limiting aldehydes and ketones is C n H 2 n O. The suffix –al is present in the name of aldehydes.
The simplest representatives of aldehydes are formaldehyde (formaldehyde) -CH 2 \u003d O, acetaldehyde (acetic aldehyde) - CH 3 -CH \u003d O. There are cyclic aldehydes, for example, cyclohexane-carbaldehyde; aromatic aldehydes have trivial names - benzaldehyde, vanillin.
The carbon atom in the carbonyl group is in a state of sp 2 hybridization and forms 3σ bonds (two C-H bonds and one C-O bond). The π-bond is formed by p-electrons of carbon and oxygen atoms. The double bond C = O is a combination of σ- and π-bonds. The electron density is shifted towards the oxygen atom.
Aldehydes are characterized by isomerism of the carbon skeleton, as well as interclass isomerism with ketones:
CH 3 -CH 2 -CH 2 -CH \u003d O (butanal);
CH 3 -CH (CH 3) -CH \u003d O (2-methylpentanal);
CH 3 -C (CH 2 -CH 3) \u003d O (methyl ethyl ketone).
Chemical properties of aldehydes
There are several reaction centers in aldehyde molecules: an electrophilic center (carbonyl carbon atom) involved in nucleophilic addition reactions; the main center is an oxygen atom with unshared electron pairs; α-CH acid center responsible for condensation reactions; S-N connection torn in oxidation reactions.
1. Addition reactions:
- water with the formation of gem-diols
R-CH \u003d O + H 2 O ↔ R-CH (OH) -OH;
- alcohols with the formation of hemiacetals
CH 3 -CH \u003d O + C 2 H 5 OH ↔CH 3 -CH (OH) -O-C 2 H 5;
- thiols with the formation of dithioacetals (in an acidic environment)
CH 3 -CH \u003d O + C 2 H 5 SH ↔ CH 3 -CH (SC 2 H 5) -SC 2 H 5 + H 2 O;
- sodium hydrosulfite with the formation of sodium α-hydroxysulfonates
C 2 H 5 -CH \u003d O + NaHSO 3 ↔ C 2 H 5 -CH (OH) -SO 3 Na;
- amines to form N-substituted imines (Schiff bases)
C 6 H 5 CH \u003d O + H 2 NC 6 H 5 ↔ C 6 H 5 CH \u003d NC 6 H 5 + H 2 O;
- hydrazines with the formation of hydrazones
CH 3 -CH \u003d O + 2 HN-NH 2 ↔ CH 3 -CH \u003d N-NH 2 + H 2 O;
- hydrocyanic acid with the formation of nitriles
CH 3 -CH \u003d O + HCN ↔ CH 3 -CH (N) -OH;
- recovery. When aldehydes react with hydrogen, primary alcohols are obtained:
R-CH \u003d O + H 2 → R-CH 2 -OH;
2. Oxidation
- the reaction of the "silver mirror" - the oxidation of aldehydes with an ammonia solution of silver oxide
R-CH \u003d O + Ag 2 O → R-CO-OH + 2Ag ↓;
- oxidation of aldehydes with copper (II) hydroxide, as a result of which a precipitate of red copper (I) oxide precipitates
CH 3 -CH \u003d O + 2Cu (OH) 2 → CH 3 -COOH + Cu 2 O ↓ + 2H 2 O;
These reactions are qualitative reactions for aldehydes.
Physical properties of aldehydes
The first representative of the homologous series of aldehydes - formaldehyde (formaldehyde) - a gaseous substance (n.o.), aldehydes of an unbranched structure and composition C 2 -C 12 - liquids, C 13 and longer - solids. The more carbon atoms a straight-chain aldehyde contains, the higher its boiling point. With an increase in the molecular weight of aldehydes, the values of their viscosity, density, and refractive index increase. Formaldehyde and acetaldehyde are able to mix with water in unlimited quantities, however, with the growth of the hydrocarbon chain, this ability of aldehydes decreases. Lower aldehydes have a pungent odor.
Obtaining aldehydes
The main methods for obtaining aldehydes:
- hydroformylation of alkenes. This reaction consists in the addition of CO and hydrogen to an alkene in the presence of carbonyls of certain Group VIII metals, for example, octacarbonyl dicobalt (Co 2 (CO) 8) The reaction is carried out by heating to 130C and a pressure of 300 atm
CH 3 -CH \u003d CH 2 + CO + H 2 → CH 3 -CH 2 -CH 2 -CH \u003d O + (CH 3) 2 CHCH \u003d O;
— hydration of alkynes. The interaction of alkynes with water occurs in the presence of mercury (II) salts and in an acidic environment:
HC≡CH + H 2 O → CH 3 -CH \u003d O;
- oxidation of primary alcohols (the reaction proceeds when heated)
CH 3 -CH 2 -OH + CuO → CH 3 -CH \u003d O + Cu + H 2 O.
Application of aldehydes
Aldehydes have found wide application as raw materials for the synthesis of various products. So, formaldehyde (large-scale production) produces various resins (phenol-formaldehyde, etc.), medications(urotropin); acetaldehyde is a raw material for the synthesis of acetic acid, ethanol, various pyridine derivatives, etc. Many aldehydes (butyric, cinnamon, etc.) are used as ingredients in perfumery.
Examples of problem solving
EXAMPLE 1
| Exercise | Bromination With n H 2 n +2 gave 9.5 g of monobromide, which, when treated with a dilute solution of NaOH, turned into an oxygen-containing compound. Its vapors with air are passed over a red-hot copper grid. When the resulting new gaseous substance was treated with an excess of an ammonia solution of Ag 2 O, 43.2 g of a precipitate was released. What hydrocarbon was taken and in what quantity, if the yield at the bromination stage is 50%, the remaining reactions proceed quantitatively. |
| Solution | We write down the equations of all occurring reactions: C n H 2n+2 + Br 2 = C n H 2n+1 Br + HBr; C n H 2n+1 Br + NaOH = C n H 2n+1 OH + NaBr; C n H 2n+1 OH → R-CH \u003d O; R-CH \u003d O + Ag 2 O → R-CO-OH + 2Ag ↓. The precipitate released in the last reaction is silver, therefore, you can find the amount of substance released silver: M(Ag) = 108 g/mol; v(Ag) \u003d m / M \u003d 43.2 / 108 \u003d 0.4 mol. According to the condition of the problem, after passing the substance obtained in reaction 2 over a hot metal grid, a gas was formed, and the only gas, aldehyde, is methanal, therefore, the initial substance is methane. CH 4 + Br 2 \u003d CH 3 Br + HBr. The amount of bromomethane substance: v (CH 3 Br) \u003d m / M \u003d 9.5/95 \u003d 0.1 mol. Then, the amount of methane substance required for a 50% yield of bromomethane is 0.2 mol. M (CH 4) \u003d 16 g / mol. Hence the mass and volume of methane: m(CH 4) = 0.2×16 = 3.2 g; V (CH 4) \u003d 0.2 × 22.4 \u003d 4.48 l. |
| Answer | Mass of methane - mass 3.2 g, volume of methane-4.48 l |
EXAMPLE 2
| Exercise | Write the reaction equations that can be used to carry out the following transformations: butene-1 → 1-bromobutane + NaOH → A - H 2 → B + OH → C + HCl → D. |
| Solution | To obtain 1-bromobutane from butene-1, it is necessary to carry out the hydrobromination reaction in the presence of peroxide compounds R 2 O 2 (the reaction proceeds against the Markovnikov rule): CH 3 -CH 2 -CH \u003d CH 2 + HBr → CH 3 -CH 2 -CH 2 -CH 2 Br. When interacting with an aqueous solution of alkali, 1-bromobutane undergoes hydrolysis with the formation of butanol-1 (A): CH 3 -CH 2 -CH 2 -CH 2 Br + NaOH → CH 3 -CH 2 -CH 2 -CH 2 OH + NaBr. Butanol-1 during dehydrogenation forms aldehyde - butanal (B): CH 3 -CH 2 -CH 2 -CH 2 OH → CH 3 -CH 2 -CH 2 -CH \u003d O. An ammonia solution of silver oxide oxidizes butanal to an ammonium salt - ammonium butyrate (C): CH 3 -CH 2 -CH 2 -CH \u003d O + OH →CH 3 -CH 2 -CH 2 -COONH 4 + 3NH 3 + 2Ag ↓ + H 2 O. Ammonium butyrate, when interacting with hydrochloric acid, forms butyric (butanoic) acid (D): CH 3 -CH 2 -CH 2 -COONH 4 + HCl → CH 3 -CH 2 -CH 2 -COOH + NH 4 Cl. |
The first group of properties is addition reactions. In the carbonyl group, between carbon and oxygen, there is a double bond, which, as you remember, consists of a sigma bond and a pi bond. In addition reactions, the pi bond breaks and two sigma bonds are formed, one with carbon and the other with oxygen. Carbon has a partial positive charge, and oxygen has a partial negative charge. Therefore, a negatively charged particle of the reagent, an anion, is attached to carbon, and a positively charged part of the molecule is attached to oxygen.
First property hydrogenation, addition of hydrogen.
The reaction takes place when heated. The hydrogenation catalyst already known to you, nickel, is used. Primary alcohols are obtained from aldehydes, secondary alcohols from ketones.
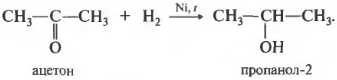
In secondary alcohols, the hydroxo group is bonded to a secondary carbon atom.
Second property hydration, water addition. This reaction is possible only for formaldehyde and acetaldehyde. Ketones do not react with water at all.
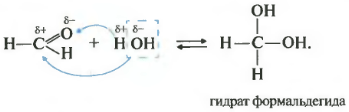
All addition reactions proceed in such a way that plus goes to minus, and minus to plus.
As you remember from the video about alcohols, the presence of two hydroxo groups on one atom is an almost impossible situation, such substances are extremely unstable. So, specifically, these two cases formaldehyde hydrate and acetaldehyde are possible, although they exist only in solution.
It is not necessary to know the reactions themselves. Most likely, the question on the exam may sound like a statement of fact, for example, they react with water and substances are listed. Among their list of which may be methanal or ethanal.
Third property addition of hydrocyanic acid.

Again, plus goes to minus, and minus to plus. Substances called hydroxynitriles are obtained. Again, the reaction itself is not common, but you need to know about this property.
Fourth property addition of alcohols.
Here again, you do not need to know the reaction equation by heart, you just need to understand that such an interaction is possible.

As usual in reactions of addition to a carbonyl group, plus to minus, and minus to plus.
Fifth property reaction with sodium hydrosulfite.

And again, the reaction is quite complicated, it is unlikely to learn it, but this is one of the qualitative reactions for aldehydes, because the resulting sodium salt precipitates. That is, in fact, you should know that aldehydes react with sodium hydrosulfite, this will be enough.
This concludes the first group of reactions. The second group is polymerization and polycondensation reactions.
2. Polymerization and polycondensation of aldehydes
You are familiar with polymerization: polyethylene, butadiene and isoprene rubbers, polyvinyl chloride are the products of combining many molecules (monomers) into one large, into a single polymer chain. That is, one product is obtained. During polycondensation, the same thing happens, but in addition to the polymer, low molecular weight products, such as water, are also obtained. That is, there are two products.
So, sixth property polymerization. Ketones do not enter into these reactions; only the polymerization of formaldehyde is of industrial importance.
The pi bond breaks and two sigma bonds are formed with neighboring monomers. It turns out polyformaldehyde, also called paraform. Most likely, the question on the exam may sound like this: substances enter into the polymerization reaction. And a list of substances is given, among which there may be formaldehyde.
The seventh property is polycondensation. Once again: during polycondensation, in addition to the polymer, a low-molecular compound is also obtained, for example, water. Formaldehyde enters into such a reaction with phenol. For clarity, we first write the equation with two phenol molecules.

As a result, such a dimer is obtained and a water molecule is split off. Now we write the reaction equation in general form.

The polycondensation product is phenol-formaldehyde resin. It has a wide range of applications ranging from adhesives and varnishes to plastics and particle board components.
Now the third group of properties oxidation reactions.
3. Oxidation of aldehydes and ketones
Eighth reaction in general list is a qualitative reaction to the aldehyde group oxidation with an ammonia solution of silver oxide. Silver mirror reaction. I will say right away that ketones do not enter into this reaction, only aldehydes.

The aldehyde group is oxidized to a carboxyl, acidic group, but in the presence of ammonia, which is a base, a neutralization reaction immediately occurs and a salt, ammonium acetate, is obtained. The silver precipitates, coating the inside of the tube and creating a mirror-like surface. This reaction occurs on the exam all the time.
By the way, the same reaction is qualitative for other substances that have an aldehyde group, for example, formic acid and its salts, as well as glucose.
ninth the reaction is also qualitative for the aldehyde group oxidation with freshly precipitated copper hydroxide two. Here, too, I note that ketones do not enter into this reaction.

Visually, the formation of a yellow precipitate will be observed first, which then turns red. In some textbooks, there is information that copper hydroxide is first formed alone, which has yellow, which then decomposes into red copper oxide alone and water. So this is not true according to the latest data, in the process of precipitation, the size of copper oxide particles changes, which ultimately reach sizes that are painted exactly in red. The aldehyde is oxidized to the corresponding carboxylic acid. The reaction occurs on the exam very often.
The tenth reaction is the oxidation of aldehydes with an acidified solution of potassium permanganate when heated.
Discoloration of the solution occurs. The aldehyde group is oxidized to a carboxyl group, that is, the aldehyde is oxidized to the corresponding acid. For ketones, this reaction has no practical meaning, since the destruction of the molecule occurs and the result is a mixture of products.
It is important to note that formic aldehyde, formaldehyde, oxidizes to carbon dioxide, because the corresponding formic acid itself is not resistant to strong oxidizing agents.
As a result, carbon goes from oxidation state 0 to oxidation state +4. Let me remind you that methanol, as a rule, under such conditions is oxidized to the maximum to CO 2, skipping the stage of both aldehyde and acid. This feature must be remembered.
Eleventh reaction combustion, complete oxidation. Both aldehydes and ketones burn to carbon dioxide and water.
Let us write the reaction equation in general form.
According to the law of conservation of mass, there should be as many atoms on the left as there are atoms on the right. Because in fact chemical reactions atoms do not go anywhere, but the order of bonds between them simply changes. So there will be as many carbon dioxide molecules as there are carbon atoms in a molecule of a carbonyl compound, since the molecule contains one carbon atom. That is n CO 2 molecules. There will be half as many water molecules as hydrogen atoms, that is, 2n / 2, which means just n.
There are the same number of oxygen atoms on the left and on the right. On the right, there are 2n of them from carbon dioxide, because each molecule has two oxygen atoms, plus n of water, for a total of 3n. On the left, there are the same number of oxygen atoms 3n, but one of the atoms is in the aldehyde molecule, which means it must be subtracted from the total to get the number of atoms per molecular oxygen. It turns out that 3n-1 atoms contain molecular oxygen, which means there are 2 times fewer molecules, because one molecule contains 2 atoms. That is (3n-1)/2 oxygen molecules.
Thus, we have compiled the equation for the combustion of carbonyl compounds in a general form.
And finally twelfth property related to substitution reactions halogenation at the alpha carbon atom. Let us turn once again to the structure of the aldehyde molecule. Oxygen pulls electron density onto itself, creating a partial positive charge on carbon. The methyl group tries to compensate for this positive charge by shifting electrons from hydrogen to it along a chain of sigma bonds. The carbon-hydrogen bond becomes more polar and the hydrogen breaks off more easily when attacked with a reagent. This effect is observed only for the alpha carbon atom, that is, the atom following the aldehyde group, regardless of the length of the hydrocarbon radical.
Thus, it is possible to obtain, for example, 2-chloroacetaldehyde. Further substitution of hydrogen atoms to trichloroethane is possible.
Aldehydes and ketones are derivatives of hydrocarbons containing one or more carbonyl groups $C = O$ (oxo groups). Aldehydes are compounds in which the carbonyl group is connected to a hydrocarbon residue and hydrogen, ketones - if it is connected to two hydrocarbon residues (the group $C = O$ is also called a keto group):
Aldehydes and ketones belong to the group of carbonyl compounds.
Depending on the structure of the hydrocarbon radical, aldehydes and ketones are divided into aliphatic, alicyclic and aromatic. Among aliphatic aldehydes and ketones, saturated and unsaturated are distinguished.
The isomerism of aldehydes is associated with the structure of the hydrocarbon residue, and ketones - in addition to the position $C = O$ of the group.
Physical properties
Definition 1
Saturated aldehydes and ketones are colorless liquids, except for formaldehyde, which is a gas under normal conditions. They are characterized by a pungent odor. Their boiling points are lower than those of alcohols, since hydrogen bonding is not typical for aldehydes and ketones, and ketones boil at more high temperature than aldehydes with the same number of carbon atoms.
Formic and acetic aldehydes, as well as ketones with a small molecular weight, are soluble in water. As the molecular weight increases, the solubility of these substances in water decreases. All aldehydes and ketones dissolve well in organic solvents (alcohol, ether, etc.).
It is believed that the carbonyl group is an osmophore, that is, a carrier of odor. Formic aldehyde has a rather pungent odor. Other lower aldehydes have a suffocating odor which, when strongly diluted, becomes pleasant and resembles the smell of vegetables and fruits. Ketones smell pretty good.
Electronic structure of the carbonyl group
Due to the different electronegativity of carbon and oxygen atoms, the carbonyl group has a high polarity (μ $\sim$ $2.5 D$ for aldehydes and $2.7 D$ for ketones) and significant polarizability. For example, the value of molecular refraction $MR$ for an oxo group is approximately 3.4, while for a single $C-O$ bond it is only 1.5.
The double bond of the carbonyl group consists, as for alkenes, of σ- and π-bonds:
Figure 2. Double bond of the carbonyl group. Author24 - online exchange of student papers
The peculiarity of the carbonyl group lies in the noticeable difference in the electronegativity of the atoms that form it. The oxygen atom has external structure$1s^22s^22p^4$ with distribution of 4 $p$-electrons over individual $x,y,z$ sublevels, but the problem of its hybridization has not been finally solved.
The existence of non-equivalent hybrid orbitals with a significant $p$-character of the type $s^n p^m$ is assumed, where $n$ tends to 1, $m$ tends to 2, that is, the $C-O$ σ-bond is most likely formed by overlapping $sp^(2_-)$ hybrid orbital of carbon and $2p_x - AO$ of oxygen. The $n$ bond is formed by the interaction of unhybridized $2p_x - AO$ carbon and $2p_x - AO$ oxygen.
The two residual pairs of $n$-electrons $2s^2$ and $(2p^2)_y$ of the oxygen atom are essentially Chemical properties carbonyl group is not affected.
Below is the structure of the simplest aldehyde - formaldehyde with data on bond angles and bond lengths.
Figure 3. Structure of the simplest aldehyde. Author24 - online exchange of student papers
bond length, $C=O$ 1.203 $C-H$ 1.101
bond angle, $()^\circ$ $H-C=O$ 121.8 $H-C-H$ 116.5
Due to the polarity of the $C = O$ bonds, the carbon atom acquires a positive effective charge, and it is called an electrophilic center, and oxygen - a negative charge, and it is called a nucleophilic center. Therefore, the carbon atom interacts with nucleophiles, which is the main interaction of the $C=O$-group of aldehydes and ketones in chemical reactions, and oxygen interacts with electrophiles. Acceptor substituents, which increase the positive charge on the carbon atom of the carbonyl group, greatly increase its reactivity. The opposite effect is observed with the donor action of substituents:
Figure 4. Donor action of substituents. Author24 - online exchange of student papers
So, aldehydes and ketones, on the one hand, exhibit significant electrophilic properties, and on the other, weak nucleophilic properties, like alcohols and ethers.
Aldehydes are more reactive than ketones as a result of two main factors. First, in the presence of the second hydrocarbon residue $R$, steric hindrance arises when the nucleophile attacks the electrophilic center. Secondly, the $R$ substituent with the $+I$ effect reduces the positive charge on the electrophilic carbon atom of the carbonyl group and increases the negative charge on the oxygen atom. As a result, the ability of the carbonyl group to react with nucleophilic reagents is weakened.
The bond energy $C = O$ is 680-760 kJ/mol (for comparison, the double bond energy $E_(C=C)$ is 590-640 kJ/mol), but due to the high polarity and polarizability, the carbonyl group is more reactive than carbon -carbon multiple bond.
Spectral characteristics of aldehydes and ketones
In UV spectra, carbonyl compounds have an intense absorption band of -185 nm due to π-π -transition and low intensity 270-300 nm due to n-π-transition:
Figure 5. UV spectra: benzaldehyde (I), aniline (II) and fluorobenzene (III). Author24 - online exchange of student papers
In the IR region of the spectrum, intense stretching vibrations of the $v_(C=O)$ carbonyl group are observed in the range of 1850-1650 cm $^(-1)$, so IR spectroscopy is a reliable method for its determination.
In the case of NMR spectroscopy for the aldehyde group, there is a characteristic proton signal at 8.5-11.0 ppm, which is also a reliable criterion for its presence in the carbonyl group.